Episode Transcript
[00:00:00] Speaker A: Foreign.
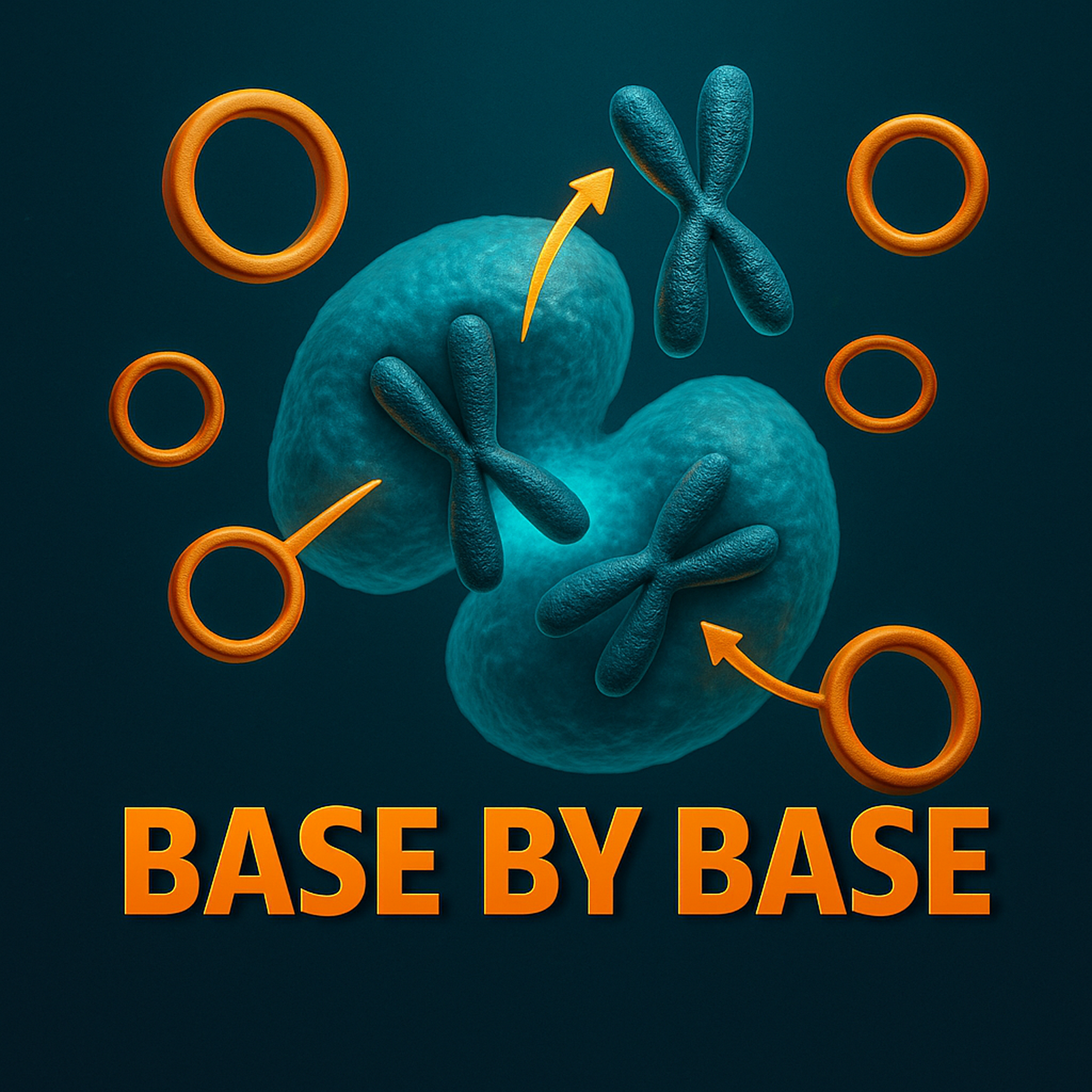
Welcome to Base by Bass, the papercast that brings genomics to you wherever you are. Thanks for listening and don't forget to follow and rate us in your podcast app. Today, we're diving into a. A really major mystery. It's a puzzle in cancer genomics that's been around for decades. I mean, think about cell division. It's this incredibly meticulous process, right? DNA gets packaged into chromosomes and this whole complex structure, the mitotic spindle, grabs them and pulls them apart so perfectly. The goal is that each new daughter cell gets an identical copy of the genome. But some of the most aggressive cancers, well, they seem to cheat. They use these. These rogue circular pieces of DNA. They're called extra chromosomal DNA, or XEDNA for short. And these things are just packed with. With potent oncogenes. We're talking things like MYC or egfr. They're basically the engines that drive drug resistance and really aggressive tumor growth. But here's the paradox, the thing that has had scientists stumped for so long. This X DNA is eccentric, meaning it has no centromere. Exactly. It's missing that essential handle, that anchor point. The cell needs to pull chromosomes apart correctly, right?
[00:01:19] Speaker B: So by all the rules of biology, it should just be lost. It should get diluted out of the cell population in just a few divisions.
[00:01:26] Speaker A: It should be gone, but it's not. It sticks around generation after generation, making the tumor more and more aggressive.
[00:01:34] Speaker B: So the question that's been lingering for what, 40 years?
[00:01:37] Speaker A: Over 40 years, yeah. How does this rogue DNA manage to hitchhike its way into every single daughter cell and secure its own sort of immortality?
[00:01:48] Speaker B: And that is the huge barrier this new research just completely smashes through. We're unpacking a deep dive that in, you know, it doesn't just identify the specific human DNA sequences. They call them retention elements that let this happen.
[00:02:00] Speaker A: It does more.
[00:02:01] Speaker B: It shows us how to switch the whole mechanism off. I mean, this is a potential game changer.
[00:02:05] Speaker A: It really is. But before we get into the details, we have to acknowledge the team that did this work.
[00:02:09] Speaker B: Absolutely. Today we celebrate the work of Venkat Sankar, King El Hung, Aditi Nanasekar and all their colleagues. It's a huge effort, mainly out of Stanford University, but with some really key contributions from places like charity, Universitatz Medizin Berlin.
[00:02:24] Speaker A: And this whole deep dive is built around their paper. Genetic elements promote retention of extrachromosomal DNA in cancer cells, which was accepted in Nature in October of 2025.
[00:02:36] Speaker B: So let's set the stage a bit more. You said X DNA drives rapid evolution in a tumor. Why is its structure being circular and missing that centromere? Why does that make it so dangerous?
[00:02:46] Speaker A: Well, these X DNA molecules are huge, first of all, megabases in size. And because they don't have that centromere, they segregate stochastically.
[00:02:54] Speaker B: Randomly.
[00:02:55] Speaker A: Randomly, yeah. So when a cell divides, the two daughter cells get unequal amounts of these ixcdna rings.
[00:03:01] Speaker B: Ah, so one cell might get a huge dose of an oncogene.
[00:03:04] Speaker A: Exactly. And that cell might suddenly become super resistant to a chemotherapy drug. This process creates what we call intraclonal heterogeneity. The tumor becomes this, this incredibly diverse population that's always evolving.
[00:03:17] Speaker B: It's basically a genetic lottery that's rigged to accelerate resistance.
[00:03:21] Speaker A: Perfectly put. And that gets back to the main problem. During mitosis, the nuclear envelope dissolves. And any DNA that's eccentric, if it's not anchored to something, it just, it floats free and gets lost or destroyed.
[00:03:31] Speaker B: Right. It gets degraded in the cytoplasm or walled off in these little things called micronuclei, where it's silenced. So for the cancer to thrive, it has to solve this inheritance problem.
[00:03:41] Speaker A: Which brings us to an idea the researchers used as a starting point. The viral analogy.
[00:03:46] Speaker B: Yes. This isn't a new trick in biology. It's actually an ancient one. Viral episomes, like from papillomavirus or Epskin Barr virus, ebv, they do the same thing. They're also eccentric.
[00:03:57] Speaker A: So how do they survive?
[00:03:58] Speaker B: They physically tether themselves to the host's mitotic chromosomes. They use their own special viral DNA elements, like the ORAP sequence in EBV and viral proteins to basically grab onto host proteins that are already sitting on the chromosomes.
[00:04:13] Speaker A: It's a dedicated viral mechanism for hitchhiking, very specialized one.
[00:04:17] Speaker B: So the burning question for cancer was, is, is Exedna using a similar trick, is there some kind of endogenous human sequence that does the same job?
[00:04:25] Speaker A: Is there a built in retention element?
[00:04:27] Speaker B: Precisely. And the challenge wasn't just thinking it might exist. It was how on earth do you find it. You're looking for a tiny functional sequence in a genome of 3 billion base pairs.
[00:04:40] Speaker A: It's an astronomical needle in a haystack problem. So they couldn't just search for a sequence.
[00:04:45] Speaker B: No, they needed a new tool, something designed to hunt for the function of retention itself.
[00:04:49] Speaker A: And this is where the paper gets really clever.
[00:04:51] Speaker B: This is where the genius comes in. They developed this incredible high throughput functional assay they called retainsec it's like a giant genomic filter.
[00:05:00] Speaker A: Okay, walk us through it. How does RetainSec work?
[00:05:02] Speaker B: The idea is simple, but the scale is huge. They started with basic bacterial plasmids. These are circular, eccentric DNA. So they're a good structural mimic for X DNA.
[00:05:13] Speaker A: Okay.
[00:05:13] Speaker B: Then they created a massive library by chopping up the entire human genome into random fragments and. And inserting one fragment into each plasmid.
[00:05:22] Speaker A: So you have millions of these little test circles, each one carrying a different piece of human DNA. A different candidate.
[00:05:28] Speaker B: Exactly. Then they took this huge pool of plasmids and transfected them into different cancer cell lines. Some that are known to have ECK DNA, like Keralo320DM and a control cell line that doesn't.
[00:05:41] Speaker A: And then comes the real test, Right, the passaging.
[00:05:44] Speaker B: Yes, serial passaging. They just let the cells grow and divide for about two weeks.
This creates immense selective pressure because any.
[00:05:52] Speaker A: Plasmid with a useless piece of DNA would just get lost during division. It would be diluted out, it would be gone.
[00:05:58] Speaker B: But, and here's the key. If a plasmid happened to contain a true functional retention element, it would successfully hitchhike on the chromosomes and it would.
[00:06:08] Speaker A: Persist and become enriched in the population. It's literally survival of the fittest sequence.
[00:06:12] Speaker B: That's it. So after all the soldavishes, they just collected the plasmids that were left and sequenced the human DNA fragments inside them. By definition, those were the retention elements.
[00:06:21] Speaker A: But first they had to prove the assay actually worked, that it was really finding a retention function.
[00:06:26] Speaker B: A great point. They used a fantastic positive control. They took the known EBV tethering sequence, or abap, put it on a plasmid and ran the assay.
[00:06:36] Speaker A: And what happened?
[00:06:37] Speaker B: It was only enriched in the EBV positive cells, the cells that actually have the viral proteins needed to make the tethering work.
It was perfect confirmation, but that the assay was specific for retention.
[00:06:49] Speaker A: That's so clean. And they also visualized this, didn't they, with live cell imaging?
[00:06:53] Speaker B: They did. They actually watched these little episomes during mitosis and they found that without a retention element, an episome would fail to segregate correctly. It would get left behind. In about 25% of cell divisions, one.
[00:07:06] Speaker A: In four divisions, it's lost a huge loss rate.
[00:07:08] Speaker B: But then they added just a single one of the retention elements, they found the failure rate plummeted. It went from 25% all the way down to just 10.4.
Wow.
[00:07:19] Speaker A: That is a massive difference in inheritance over dozens of generations. That's the difference between an oncogene disappearing and an oncogene taking over the tumor.
[00:07:27] Speaker B: It's the key to its immortality.
[00:07:29] Speaker A: Okay, so the big reveal after all this filtering, what did these retention elements, these res, actually look like?
[00:07:36] Speaker B: They were not random at all. The thousands of res they pulled out were almost all a very specific type of sequence. CPG rich gene promoters, and bits of five prime untranslated regions, or UTRs.
[00:07:50] Speaker A: So active regulatory parts of the genome. That's a huge clue right there. And they found that the effect was cumulative.
[00:07:57] Speaker B: Yes, that was another fascinating part. They tested plasmids with 1, 2, or even 3 copies of an re. And the more copies they added, the better the retention. It's an additive effect, which is so.
[00:08:07] Speaker A: Different from a centromere. You only want one of those.
[00:08:09] Speaker B: Exactly. Having more than one centromere is a catastrophe for a chromosome. But here, more res means more tethers, which means a higher chance of being inherited. It's a completely different logic.
[00:08:19] Speaker A: So we know what they are. What about their echogenetic state? What did the chromatin look like at these spots?
[00:08:25] Speaker B: They found these res were strongly associated with active chromatin. They were loaded with active histone marks, things like H3K27AXI and H3K4ME3, and lots of active RNA polymeraseq.
[00:08:36] Speaker A: So the parts of the ECDNA that are being expressed as oncogenes are the same parts responsible for its inheritance.
[00:08:42] Speaker B: The two functions are physically linked. Survival and activity go hand in hand.
[00:08:47] Speaker A: Okay, but how did they prove that these aries were actually, you know, physically touching the chromosomes during that chaotic division phase.
[00:08:55] Speaker B: For that, they used a technique called HI C. It's a method for mapping all the physical contacts DNA makes with itself. But they did it on cells that were frozen or arrested right in the middle of mitosis so they could get.
[00:09:09] Speaker A: A snapshot of the interactions. And where were the RE's docking?
[00:09:12] Speaker B: They were docking at very specific, predictable anchor points on the mitotic chromosomes. And these anchor points are defined by this really cool process called mitotic bookmarking.
[00:09:22] Speaker A: Mitotic bookmarking. That's a fantastic phrase for the listener. What is that exactly?
[00:09:27] Speaker B: Yeah, it's a great term. So imagine when the cell divides, it has to pack all its DNA down incredibly tightly. Most of the proteins that regulate genes get kicked off. But mitotic bookmark bookmarks are like the little sticky notes. There's specific spots on the DNA that stay bound by key proteins like BRD4 or the sweat SNF complex all the way through mitosis. They bookmark the important genes so the daughter cells know which ones to turn on again right away.
[00:09:53] Speaker A: So the SCDNA isn't just grabbing on anywhere. It's targeting the most important, most stable regulatory hotspots that the cell itself has prioritized for inheritance.
[00:10:04] Speaker B: It's hijacking the cell's own memory system. And the researchers made an even deeper.
[00:10:08] Speaker A: Connection about the logic of it all.
[00:10:10] Speaker B: Yes, they realized this interaction, the SCDNA's retention element grabbing onto a bookmark on a chromosome, is happening intermolecularly between two different pieces of DNA.
[00:10:20] Speaker A: Okay.
[00:10:20] Speaker B: But what it's doing is it's perfectly mimicking the promoter enhancer interactions that normally happen in cysts or along a single chromosome. Cancer is basically repurposing the fundamental language of gene regulation for its own selfish inheritance.
[00:10:33] Speaker A: That's. That is profound. And this isn't just a theory from a cell culture dish.
[00:10:38] Speaker B: Absolutely not. They went straight to patient data. They looked at whole genome sequencing from real tumors and found that a staggering 98% of all oncogene carrying XDNAs had these retention elements.
[00:10:49] Speaker A: 98%. So it's basically a universal requirement.
[00:10:52] Speaker B: It seems to be a critical evolutionary step for an X DNA to be successful. In a tumor, it looks like it has to grab an oncogene and enough of these retention elements.
[00:11:02] Speaker A: And you mentioned that this even explains the size and structure of the X DNA we see it.
[00:11:07] Speaker B: Does they notice that in parts of the genome where these aries are naturally sparse, the xdnas that form are much, much larger. The cell is forced to break off a bigger chunk of the chromosome just to make sure it captures enough of these essential aries to guarantee stable inheritance. The size isn't random. It's dictated by the need for these anchors.
[00:11:27] Speaker A: Incredible. So all of this is leading to the most important part.
A vulnerability.
They didn't just find the lock. They may have found the key.
[00:11:35] Speaker B: That's the most exciting part. They look back at the re sequence, those cpg rich promoters. These are sites for DNA methylation. The cell's off switch.
[00:11:44] Speaker A: And what did they find?
[00:11:45] Speaker B: The res on the X DNA were consistently hypomethylated. They were epigenetically on or open, which makes sense.
[00:11:52] Speaker A: That open state is probably what allows those bookmarking proteins to bind. So the obvious question becomes, what happens.
[00:11:59] Speaker B: If you force it shut?
[00:12:00] Speaker A: Right.
[00:12:00] Speaker B: So that's what they did. They used a tool called Krisprof. It's like a Surgical robot. It uses a dead Cas9 to guide a methyltransferase enzyme directly to the RE sequences on the X DNA.
[00:12:11] Speaker A: So no cutting, just writing a chemical off signal right onto the retention element. What happened?
[00:12:17] Speaker B: It completely wiped out the retention function in the glioblastoma cells they tested. Targeting the REIs with methylation caused the X DNA to become untethered. They saw it floating outside the nucleus and. And the total amount of X DNA in the nucleus just plummeted.
[00:12:30] Speaker A: That is a massive therapeutic opportunity. It proves that the open hypomesylated state is absolutely essential for this whole process.
[00:12:38] Speaker B: It is. You can essentially force the cancer to lose its most powerful weapon, the oncogenes on the X DNA, just by changing the epigenetic state of its anchors. It suggests a whole new way to.
[00:12:48] Speaker A: Think about treatment and a much more targeted one than current drugs.
[00:12:51] Speaker B: A much more targeted one. Instead of, you know, broad methylation inhibitors that have tons of side effects, you could design something to silence only these specific elements on the istna.
[00:13:02] Speaker A: Before we get to the big take home message, were there any major limitations or, you know, next steps that came out of this?
[00:13:09] Speaker B: Well, one thing they noted was redundancy. They tried knocking out single proteins that are part of that bookmarking complex, like a protein called CHD1. Knocking out just one factor didn't cause a massive untethering. This suggests that the process is really robust. It probably involves a whole complex of proteins working together.
[00:13:28] Speaker A: It's not a single point of failure. The cancer has backups.
[00:13:31] Speaker B: Exactly. So while we found the DNA sequence, the re, the next step is to figure out the full cast of protein characters that actually binds to it. That could reveal even more druggable targets.
[00:13:41] Speaker A: Okay, so let's try to sum this all up. For 40 years, we knew this rogue eccentric DNA was somehow hitchhiking through cell division, but we didn't know how.
[00:13:51] Speaker B: And now we know. The persistence of oncogenic X DNA is guaranteed by these newly discovered human sequences they call retention elements, which are basically active gene promoters.
[00:14:02] Speaker A: And the mechanism is that these elements physically tether the SCDNA to these stable mitotic bookmarks on the host chromosomes, which secures their inheritance.
[00:14:12] Speaker B: And critically, because this whole system depends on the retention element being in an on or hypomethylated state, we now have a potential way to switch it off. It offers a new path to force the cancer to lose its most dangerous DNA.
[00:14:25] Speaker A: Which leaves us with a really provocative thought for the future. What does this discovery mean for how we design the next generation of cancer therapies? Are we on the verge of shifting shifting focus from just killing cancer cells to instead preventing the very inheritance of the DNA that makes them so deadly in the first place?
[00:14:41] Speaker B: This episode was based on an Open Access article under the CCBY 4.0 license. You can find a direct link to the paper and the license in our episode description. If you enjoyed this, follow or subscribe in your podcast app and leave a five star rating. If you'd like to support our work, use the donation link in the description now. Stay with us for an original track. Created a special especially for this episode and inspired by the article you've just heard about. Thanks for listening and join us next time as we explore more science base by base.
[00:15:33] Speaker C: Hidden loops in the quiet storm Shining bright where the dark grows warm Floating pages with no spine to hold Learning how the stories unfold Signals trace through the crowded light Little circles fighting to stay inside when the bridge between the cells grows thin they anchor close so they won't give in Every fragile thread they chase Finds a place in the turn in space Silent foot bookmarks start to rise Guiding them through shifting skies Circles that refuse to fall holding fast in the break of it all Riding on the chromosomes high Keeping every copy alive in the noise of a world so small these circles refuse to fall.
Promise sparks that the cells ignite CPG lights keeping tether flight through the storms when the spindles twist they reach for marks that still persist Patterns fading when metal shadows grow Losing grip when the anchors let go but when the ties remain away the circles rise with every break Every fragile threat they chase Finds a place in the turn in space Silent book mark star to rise Guiding them through shifting skies Circles that refuse to fall holding fast in the break of it all Riding on the chrome the sun tight Keeping every copy alive in the noise of a world so small these circles the flowers refused to fall.
Marked by echoes in the maze Borrowing strength from ancient ways Bound to a spark that will not fade even as the boundaries change where the scattered pieces meet they find a rhythm in repeat.
Circles that refuse to fall holding fast in the break of it all Riding on the chromosome tight Keeping every copy alive in the noise of a world so small these circles refuse to fall this is up to you, Sam.