Episode Transcript
[00:00:00] Speaker A: Foreign.
Welcome to Base by Bass, the papercast that brings genomics to you wherever you are.
Today we're diving into, well, something pretty fundamental in human health.
Male fertility, or rather, infertility.
When couples struggle to conceive, it turns out that around, what, 19% of the time, the issue comes down to athenosospermia.
[00:00:34] Speaker B: Yeah, that's the term for sperm that just can't swim effectively. And, I mean, if sperm can't move properly, sustained forward movement, that's a complete non starter at the molecular level.
[00:00:45] Speaker A: Right. Fertilization isn't just a casual swim.
[00:00:47] Speaker B: No way. Sperm have to swim hard. They have to switch into this hyperactivation mode, navigate all these complex signals. It's intense. And if the flagellum, you know, the tail that does all the work, if its molecular engine isn't put together right, the whole thing just fails.
[00:01:01] Speaker A: We know the basics, right. The flagellum's motor gets its juice ultimately from a burst of campi, that key signaling molecule. But here's the catch, and it's a big one. The enzyme that actually makes the campi. It needs to be locked down, perfectly positioned, and stabilized. Right there in the flagellum.
[00:01:16] Speaker B: Exactly. If it's not anchored correctly, it just gets lost. It's gone. And the sperm is, well, dead in the water, literally.
[00:01:23] Speaker A: So the big question for this deep dive really is this.
[00:01:25] Speaker B: Yeah.
[00:01:26] Speaker A: What's the absolutely critical molecular anchor, the sort of chassis organizer, the thing that's totally non negotiable for keeping that swimming machinery intact.
[00:01:35] Speaker B: And the answer is kind of surprising. It's an uncharacterized protein found purely through evolutionary detective work. And its discovery forces us to completely rethink the job of another major component, an ion channel part.
[00:01:49] Speaker A: So this isn't just about understanding why some men are infertile. This gives us a really clear direct target for diagnosis and maybe even for in vitro rescue therapies. Let's unpack this breakthrough.
[00:02:01] Speaker B: Okay. So, yeah, let's give credit where it's due. First today, we're highlighting the work from a team including Rita Narita, Haruhiko Miyata, and Masahiro Ikawa. They're affiliated with institutions like Osaka University, and their work has really pushed forward our understanding of how campy signaling is regulated for sperm motility. Mm.
[00:02:19] Speaker A: And importantly, it offers some real insights into potential treatments for asinosospermia. So let's ground this clinically. First, acinosospermia, the definition is having progressive sperm motility under 32% or total motility below 40%.
[00:02:34] Speaker B: Right. And it's a significant chunk of the male infertility cases worldwide. Not a small issue.
[00:02:39] Speaker A: And scientifically we know the sort of ignition sequence starts with outside triggers, doesn't it?
[00:02:44] Speaker B: It does. When sperm get into the female reproductive tract, bicarbonate ions, HCO3, they trigger the main enzyme for flagellar signaling. That soluble adenily cyclase. You'll see it called SAC or EDCY 10.
[00:02:57] Speaker A: Right. SCC, that's the key converter. It grabs that bicarbonate signal and uses it to churn out adenosine 3O5 or cyclic monophosphate.
[00:03:06] Speaker B: Yep, CMP, that's the real power switch. It activates protein kinase PKA and PKA.
[00:03:12] Speaker A: Phosphorylation, then kicks off that really vigorous high energy movement. The stuff needed for capacitation, for actually reaching the egg.
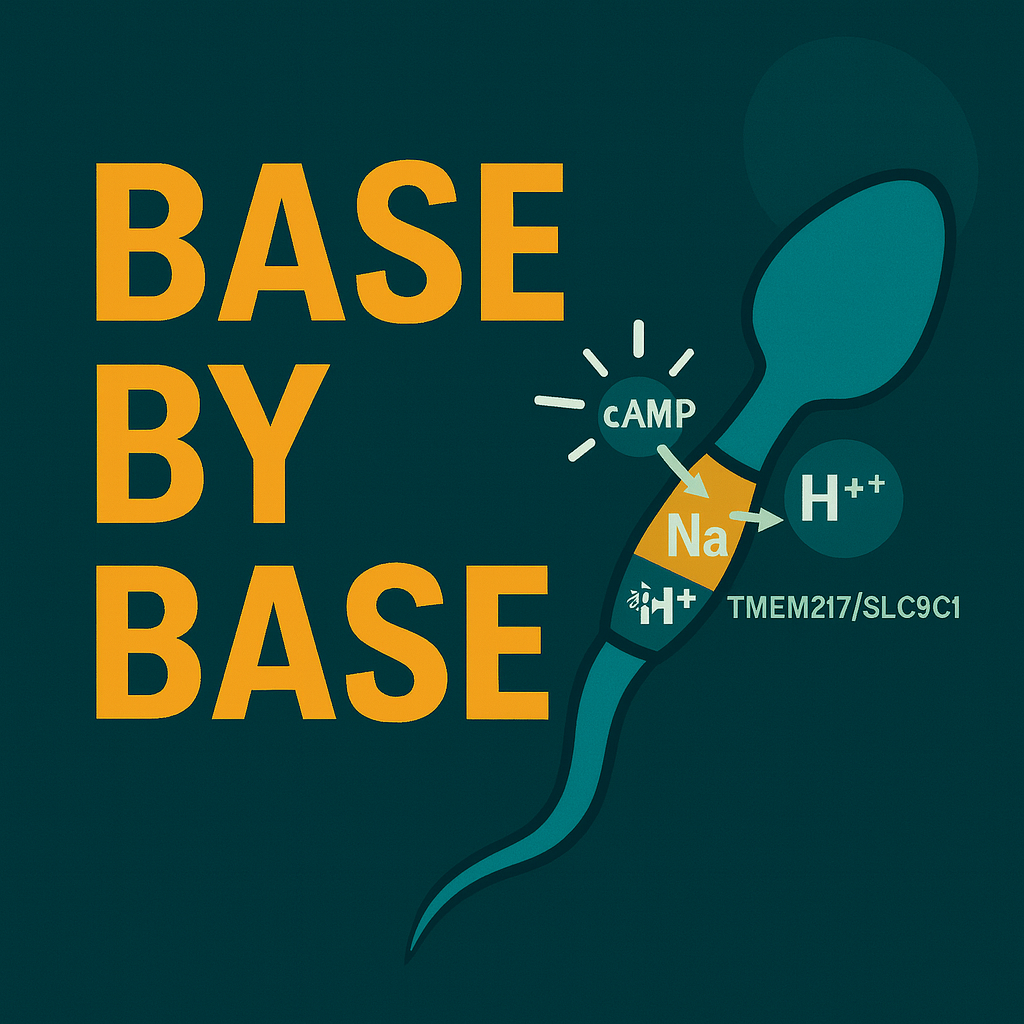
[00:03:19] Speaker B: And this is crucial, this whole camp he production thing, it depends on something else already being locked in place, and that's SLC9C1.
[00:03:25] Speaker A: Ah, the mysterious SLC9C1, the sperm specific sodium proton exchanger. Snhe sometimes call.
[00:03:33] Speaker B: Exactly. And we knew it was important. Previous knockout studies, I mean, they showed if you get rid of SLC9C1, the sperm are just immodal instantly.
[00:03:41] Speaker A: Wow.
[00:03:41] Speaker B: And critically, losing SLC9C1 directly leads to a massive drop in the amount of full length SAC protein and naturally crippled CMP production.
[00:03:51] Speaker A: So it's absolutely essential. But SLC9C1 is kind of a weird player among ion exchangers, isn't it?
[00:03:55] Speaker B: It is a bit odd. Most NAND plus H exchangers are, well, relatively standard transmembrane proteins. But SLC9C1 has this extra complex bit called a voltage sensing domain. A vsd.
[00:04:07] Speaker A: Right, the vsd. And in other animals, like sea urchins, that VSD is thought to control the ion transport based on electrical charge. Right. Voltage changes.
[00:04:14] Speaker B: Precisely. But in mammals, its job has been really unclear. Does it still sense voltage here? Or maybe it does something completely different. That was the puzzle surrounding SLC9C1.
[00:04:23] Speaker A: Okay, so they knew SLC9C1 was vital and they suspected it needed a partner to keep it stable and in the right place.
So how did they go about finding this partner? Like how do you track down a protein's buddy?
[00:04:37] Speaker B: Well, they didn't start with the usual suspects like running biochemical assays right away. They started with evolutionary genomics. They use this really sophisticated tool, phylogenetic profiling called cladioscope.
[00:04:50] Speaker A: Cladioscope sounds cool. Like an evolutionary dating profile.
[00:04:54] Speaker B: Yeah, you could kind of think of it like that. It scans genomes across tons of different species and looks for genes that always show up together. The idea is if they always co occur, they probably depend on each other functionally.
[00:05:04] Speaker A: That's smart. Letting evolution do the legwork to find linked functions. So what popped up the top hit?
[00:05:09] Speaker B: The gene that co evolved most strongly with SLC9C1 but had like zero known function, was a transmembrane protein. They ended up calling TMEM217TME217 and it looked perfect. It's highly conserved in mammals. It clearly travels with SLC9C1 through evolution. And nobody knew what it actually did.
[00:05:28] Speaker A: So the mission became prove they're linked and figure out TMM217's job.
[00:05:33] Speaker B: Exactly. So first step, generate Timem217 knockout mice. They used CRISPR Cas9 for that, creating a pretty big deletion.
[00:05:41] Speaker A: And then they threw the modern molecular biology toolkit at it, I assume.
[00:05:45] Speaker B: Oh yeah, the full suite. Including some really cutting edge stuff, like they used AlphaFold3 for structural prediction.
[00:05:51] Speaker A: Ah, so getting a computer model of how they might physically stick together even before testing it in the lab.
[00:05:56] Speaker B: Precisely. It gives you a strong hypothesis about the interaction sites.
[00:06:00] Speaker A: And you mentioned another technique, something about mass spectrometry for dealing with low protein levels.
[00:06:05] Speaker B: Right. That was key. They used data independent acquisition mass spectrometry, or DIA, specifically on mature sperm.
[00:06:12] Speaker A: Why DIA? What's the advantage there?
[00:06:14] Speaker B: Because SLC9C1, the protein they really needed to track, is actually present in super low amounts in mature sperm. It's hard to detect reliably. Standard proteomics methods, the less sensitive ones might have just missed it entirely. Or given shaky numbers. DIA let them accurately quantify these rare proteins in both the knockout and the wild type sperm.
[00:06:35] Speaker A: Crucial for proving whether the protein was actually missing or just not working, right?
[00:06:38] Speaker B: Exactly. That quantification was fundamental to their big conclusion about protein loss.
[00:06:43] Speaker A: Okay, let's get to the results, because you said they were dramatic. First off, where is this TMM217 protein actually found in the sperm?
[00:06:51] Speaker B: Well, the TMM217 gene is mostly expressed in the testes and the protein itself. It localizes exactly where you'd hope. The principal piece of the sperm flagellum. Right in the tail.
[00:07:03] Speaker A: Okay, prime location. And the consequence of knocking out TMAM217.
[00:07:08] Speaker B: Complete infertility. Just like the SLC9C1KOS. The TMM217 knockout males were identical. Phenotypically identical. Yep. They documented over 30 successful matings. They checked for vaginal plugs. So they knew mating occurred. But zero pups were born. None.
[00:07:24] Speaker A: Wow. And the reason was obvious under the microscope.
[00:07:26] Speaker B: Absolutely. The sperm were overwhelmingly immodal. Their velocity parameters, VAP, VSL, VCL. The standard measures were barely 30% of the wild type sperm. They just weren't moving properly.
[00:07:38] Speaker A: Okay. Identical phenotypes, motility, infertility. Does that mean TMM217 is just stabilizing SLC9C1? Or could it have another role too?
[00:07:50] Speaker B: That's the million dollar question, isn't it? The data really points towards the stabilization role being primary because they also saw something else. A weird physical defect. The knockout sperm showed this really distinct hairpin, like bending in the tail when they were put in isotonic media.
[00:08:05] Speaker A: A hairpin bend. What does that suggest?
[00:08:07] Speaker B: Well, that kind of shape abnormality often points towards problems with osmoregulation, how the cell handles water balance. And that links right back to the ion exchange function of SLC9C1, which is now missing. So it suggests the structure itself is compromised because the key protein isn'.
[00:08:22] Speaker A: You've got the physical failure, the bending and the functional failure, the immortality, both happening.
[00:08:26] Speaker B: Exactly. But the really big question was how TMMM217 and SLC9C1 actually connect. And the answer? Well, it changes the game for how we understand SLC9C1's structure.
[00:08:37] Speaker A: Okay, I'm listening. How do they link up?
[00:08:39] Speaker B: CO immunoprecipitation. COIP experiments confirmed it. They physically bind to each other. TME M217 directly interacts with SLC9C1. But the stunner was which part of SLC9C1 was responsible for the connection?
[00:08:54] Speaker A: Let me guess.
The voltage sensing domain. The vsd.
[00:08:59] Speaker B: You got it. The vsd. This domain. The bit everyone thought was about sensing electrical changes to open and close the channel. Turns out that's the docking site for TME M217.
When the researchers chopped off the VSD region, the binding completely disappeared. Gone.
[00:09:16] Speaker A: Whoa. So it's not about voltage sensing. At least not primarily in mammals. It's acting as a physical anchor point.
[00:09:22] Speaker B: That's what the evidence screams. It's about structure. It's about organization. The VSD is the handle that TME M217 grabs onto.
[00:09:28] Speaker A: That's a paradigm shift for VSD function in this context. The sperm tail protein is rewriting the rules. The VSD is a specialized molecular anchor.
[00:09:36] Speaker B: It is. And think about the consequences of losing that anchor. It was catastrophic for the cell in those TMEM217KO mature sperm. It wasn't just SLC9C1 that was missing. The full length functional version of SACCSFL was also completely undetectable.
[00:09:52] Speaker A: Undetectable? Not just reduced, but gone, gone, vanished.
[00:09:56] Speaker B: And the data suggests this loss happens during spermiogenesis, that final maturation stage from spermatid to spermatozone.
[00:10:03] Speaker A: So it implies a failure to either transport the complex into the flagellum correctly during development, or maybe a failure to maintain it once it's there.
[00:10:10] Speaker B: Exactly. Either way, the whole SLC9C1SAC complex fails to establish itself properly in the tail. And without that complex, no KMP production. Right. Intracellular CMP levels absolutely plummeted, which in turn just crippled the downstream signaling. The PKA activation, the phosphotyrosine cascades, everything needed for motility and capacitation.
[00:10:32] Speaker A: It's such a clear chain reaction. No TMM217 anchor, no SLC9C1 SCC complex, no campy, no swimming, no fertilization.
[00:10:40] Speaker B: Perfect storm of molecular failure leading to infertility.
[00:10:43] Speaker A: Okay, but if the ultimate problem is just the lack of CAMP downstream, could you potentially bypass that missing anchor? Like artificially boost camp levels?
[00:10:52] Speaker B: That's the crucial next step. The translational part. And yes, they tried exactly that. They took the immobile KO sperm and treated them with a cell permeable CAMP analog. It's called dbcmp, along with an inhibitor, ibmx, which stops CMP breakdown.
[00:11:08] Speaker A: And did it work? Did they start swimming again?
[00:11:10] Speaker B: It took a little while, but yes. After about 120 minutes of incubation, sperm motility was substantially recovered. Not perfect maybe, but way, way better.
[00:11:18] Speaker A: That's huge. But the real test is fertilization, isn't it? Could these rescued sperm actually make pups?
[00:11:24] Speaker B: They could.
Using IVF in vitro fertilization, the fertilizing ability of the 10M217KO sperm was fully restored.
[00:11:32] Speaker A: Fully restored. Wow.
[00:11:33] Speaker B: Yeah, they got 28 healthy pups born from these rescued sperm.
Now, critically, it wasn't just TMP analogs. They also needed to use specialized IVF media.
[00:11:42] Speaker A: What kind of media?
[00:11:43] Speaker B: Media containing compounds like MBCD and gsh. These are known to help with things like sperm plasma membrane fluidity, sort of priming the sperm for fertilization in the artificial environment.
[00:11:53] Speaker A: Okay, so it needed a bit of extra help, but the core KMP deficit was the key block.
[00:11:58] Speaker B: Absolutely. It confirms the infertility phenotype is driven specifically by that lack of CAM P signaling which results directly from that missing physical complex.
[00:12:07] Speaker A: So thinking about TMM 217's role, then it's not an Ion channel itself. It's not an enzyme, it's purely structural stabilizer.
[00:12:15] Speaker B: Its primary role seems to be logistics. Yeah, it's the essential scaffold, the anchor protein. It makes sure SLC9C1 and therefore SACFL ends up in the right place, the flagellum, and stays there. Without that TMEM217 anchor, the complex either gets lost, degraded, or maybe never even makes it into the tail properly during assembly.
[00:12:35] Speaker A: And let's circle back to that VSD finding because it's so striking. For years voltage sensing domains meant well, sensing voltage, dynamic regulation, open and closing channel.
[00:12:45] Speaker B: Right, that was the dogma.
[00:12:46] Speaker A: But here in mammalian sturm, it looks like it's been repurposed. Co opted for a purely structural job holding onto TMMO2 Pimentine. It's an organizational hub, not a dynamic sensor.
[00:12:55] Speaker B: It really reframes how we might think about VSDs in other contexts too, potentially. And if you zoom out to the evolutionary picture, TMM217 is really well conserved in mammals, but you don't find it in most invertebrates.
[00:13:08] Speaker A: Ah, interesting. So what does that suggest?
[00:13:10] Speaker B: It suggests that maybe as mammalian reproduction evolved, needing this really complex powerful flagellar movement, perhaps in a different biochemical environment, a new specialized anchor system involving TMM217 became necessary. Necessary to make sure that ancient SAC camp engine was properly installed and regulated within this more intricate mammalian sperm struct.
[00:13:35] Speaker A: That has really significant clinical implications, doesn't it? We now have a brand new candidate gene, TMM217.
[00:13:40] Speaker B: Exactly. If some cases of human Asthenosisomia are caused by mutations, pathogenic variants in TMM217 or maybe affecting its interaction with SLC9C1, we now know exactly what pathway to investigate diagnostically.
[00:13:52] Speaker A: Look for broken anchors.
[00:13:53] Speaker B: Look for broken anchors. Precisely. And even better, we potentially have a treatment pathway ready to go. The fact that they could rescue fertility in vitro with CMP analogs and that optimized media, that's an immediate promising strategy for men with infertility linked specifically to these KMMP pathway defects.
[00:14:10] Speaker A: It offers real hope, especially for those maybe currently diagnosed with idiopathic or unexplained infertility.
[00:14:17] Speaker B: It really does.
[00:14:17] Speaker A: But like all good studies, it also points to the next questions. Right. The paper mentions connections between TMEM217 and IFT proteins intra slagellar transport.
[00:14:28] Speaker B: Yeah, IFT is the machinery that literally carries cargo up and down the flagellum like a molecular railway. So the next step is figuring out the logistics. How does TMEM 217 actually work with IFT to get the whole SLC9C1SAC complex transported into the tail during development. Is TME M217 helping load the cargo? Is it the destination sign? We don't know the details yet.
[00:14:50] Speaker A: Right. What about that weird hairpin band? Does that happen in the SLC9 C1 knockouts too? Comparing those structural defects seems important.
[00:14:56] Speaker B: Absolutely. Understanding that interplay between the structural integrity, the missing ion regulation and the CAM P signaling, there's still more to uncover there. But the take home message here is pretty clear, I think. That interaction between TMMM217 and the Sodium Proton Exchanger SLC9C1, it's an absolutely non negotiable step. It's required to build the sperm's engine correctly. TMM217 uses that unique voltage sensing domain on SLC9C1 basically as a handle, an anchor point, and that ensures SLC9C1 and its partner SAC are there to drive Chem MP signaling. If you lose that anchor complex, you get infertility, at least in mice.
[00:15:35] Speaker A: So here's a thought to leave you if this TMMM217 scaffolding system seems to be a later mammalian evolutionary addition, what specific pressures in mammalian reproduction, maybe compared to other vertebrates or invertebrates, made it so essential to develop this specific anchoring mechanism for such a fundamental process?
[00:15:53] Speaker B: Hmm, that's a great question. What was different?
[00:15:55] Speaker A: Something for you to mull over as you think about the incredible molecular engineering needed just to get one cell moving.
[00:16:01] Speaker B: Definitely something to ponder.
[00:16:03] Speaker A: This episode was based on an Open Access article under the CC BY 4.0 license. You can find a direct link to the paper and the license in our episode description if you enjoyed this, follow or subscribe in your podcast app and leave a five star rating. If you'd like to support our work, use the donation link in the description. Thanks for listening and join us next time as we explore more science base by base.
Sam.