Episode Transcript
[00:00:14] Speaker A: Welcome to Base by base, the papercast that brings genomics to you wherever you are. If you received a genetic test result for, say, a critical hereditary cancer gene, how would you feel if the most definitive medical guidance you got back was simply.
We don't know, it sounds terrifying, right? But that's the reality. For countless patients. Their genetic reports come back flagged with something called a variant of uncertain significance or a vus.
Basically, these are changes in DNA we can see, but we just can't confidently say if they're harmless or if they're genuinely pathogenic disease causing.
[00:00:48] Speaker B: And a major source of these vuss, especially in hereditary cancer genes, comes down to problems with pre MRNA splicing. You know that crucial process where the cell cuts out the non coding bits, the introns, and stitches the coding exons back together perfectly.
[00:01:02] Speaker A: Yeah.
[00:01:02] Speaker B: It's surprisingly easy for subtle mutations to mess that whole process up, often silently.
[00:01:07] Speaker A: Yeah. And our deep dive today is focusing on one gene where this uncertainty is a huge problem. CHK2. It's linked to a moderate but definitely significant risk for breast, prostate and colorectal cancers. And when a C H E K2 variant gets labeled a VUS, well, it basically paralyzes clinical action. It really limits the power of genetic testing to guide preventative care.
[00:01:26] Speaker B: Yes, exact. Exactly. Clinical limbo for a gene linked to cancer risk is.
Well, this is not acceptable. So today we're looking at how a big international research effort used a really smart functional approach, the minigene system, to basically pull dozens of these confusing Q2 splicing variants out of that VUS category and into clearer actionable classifications. Yeah. Okay, let's unpack this. But first, before we really dive into the nitty gritty science, we absolutely have to acknowledge the sheer scale of this work. I mean, this wasn't just one lab. It was a multi institutional team effort across Spain, the UK and the Netherlands. And they weren't just pulling variants out of thin air. They were using data from the massive breast cancer after diagnostic gene sequencing project, that's bridges. It gave them genetic info from over 60,000 breast cancer cases and more than 50,000 controls. Huge numbers. So today we really celebrate the work of these teams. They've seriously advanced our understanding of how these splicing defects contribute to cancer risk.
[00:02:23] Speaker A: Okay, let's set the clinical stage a bit more. Why is Qique 2 specifically such an important gene to sort out?
[00:02:29] Speaker B: Well, the QIQUE2 gene, it codes for a protein called checkpoint kinase 2.
Think of it as a key guardian of your genome's integrity. If There's DNA damage, like a double strain break. CHK2 steps in right away to help manage the response. It's really essential, absolutely essential.
[00:02:46] Speaker A: And its clinical relevance, particularly in breast cancer, is immense. We know for sure that variants that truncate the protein, stop it from being made properly called PTVs, are linked to breast cancer risk. In fact, Chiche2 accounts for almost a quarter of all PTVs found across the main eight hereditary breast cancer genes. It's second only to BRCA2.
[00:03:05] Speaker B: Right. And while the risk isn't as high as, say, BRCA1, it's considered moderate, maybe around a 25% absolute risk by age 80. The guidelines for carriers are still quite proactive. They recommend annual mammograms starting at 40 and breast MRIs maybe even starting at 30 or 35. In that kind of screening, it's estimated it could reduce breast cancer mortality by something like 58%. 58%. That's a massive potential benefit. But here's the huge roadblock.
Researchers looked at Clinvar, you know, the big public database of variants, and found something like 54% of all reported CTK2 variants were stuck as the US or had conflicting interpretations over half.
So for most carriers, that genetic test result is just clinically useless for making.
[00:03:48] Speaker A: Decisions and trying to figure out these splicing problems. Traditionally, using patient rna, that's been a real headache, hasn't it? Why is it so complicated?
[00:03:56] Speaker B: Yeah, it's tough. It's usually a mix of things. First, getting the right samples can be hard. You don't always have access to fresh RNA from the relevant tissue. But maybe more importantly, when you test a patient directly, you're looking at both copies of the gene, the normal one, the wild type and the variant one. And often the signal from the normal healthy allele just swamps everything else. It makes it almost impossible to see, let alone quantify, the effect of the mutant allele.
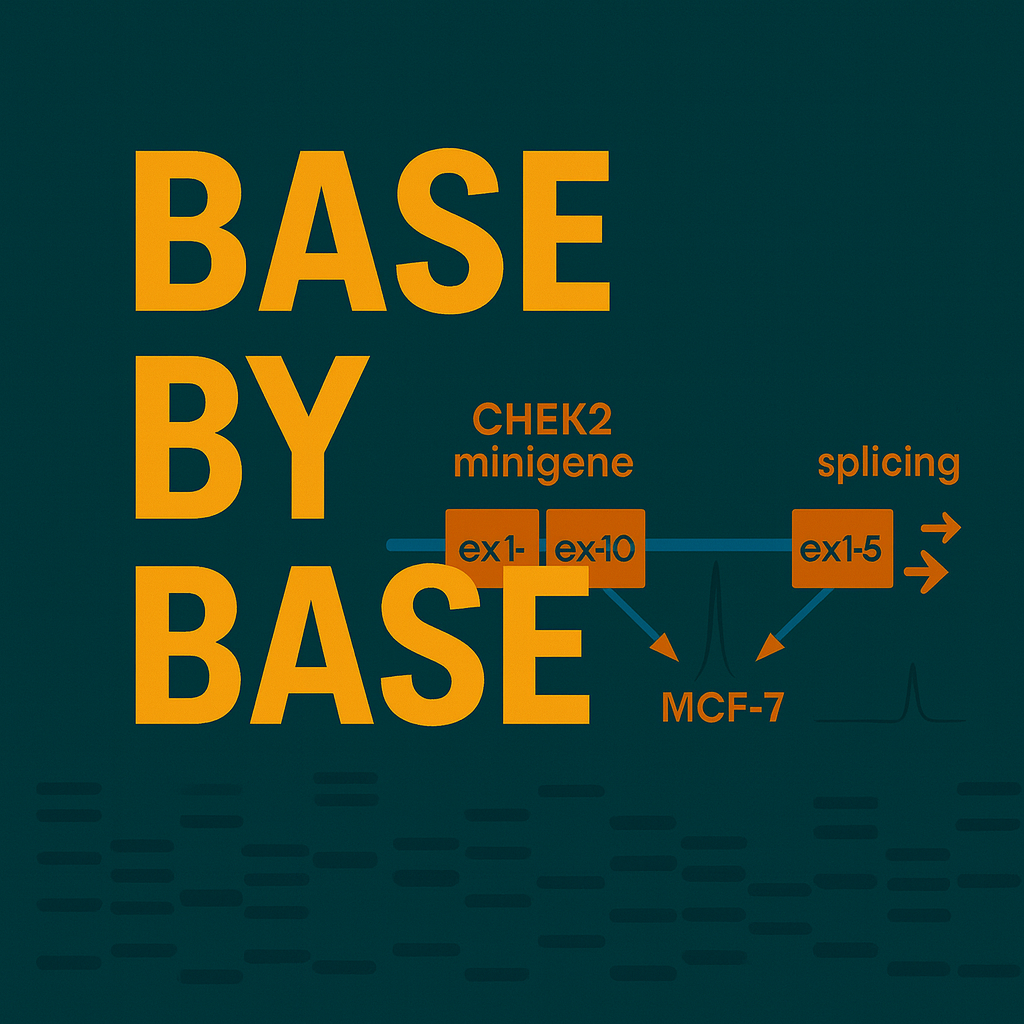
[00:04:19] Speaker A: Okay, so that brings us neatly to the clever solution in this paper, the minigene approach. They started with A list of 128 potential cheat2splice site variants identified in that huge Bridges dataset. But they didn't just test everything blindly, they were smart about it. They used computational tools first, specifically Max and Scan, to predict which marions were most likely to actually mess up splicing. That helped them sort of triage the list and focus on 52 top candidates.
[00:04:46] Speaker B: Right, and here's where the real innovation comes in. They built three different CHEAT two minigenes. Basically, these are smaller, manageable versions of the gene, covering all 15 exons packaged into a special vector called PSA.
And the really key thing here is that each minigene represents only one allele, the variant allele. So straight away you eliminate all that confusing noise from the normal copy of the gene.
[00:05:07] Speaker A: Clever. And they put these minigenes into MCF7 cells. Why the specific cells?
[00:05:12] Speaker B: Ah, good question. That's actually a really important detail for making the results relevant.
MCF7 cells are a human breast cancer cell line. Since breast cancer is the main risk associated with GK2, using these cells means the experiment is happening in a context that has the right cellular machinery, the right splicing factors, RNA binding proteins, everything.
It makes the findings much more physiologically meaningful than if they just used some generic lab cell line.
[00:05:39] Speaker A: Okay, that makes sense. And they used a high resolution fluorescent RT PCR method to analyze the results.
Why was that important?
[00:05:47] Speaker B: That allowed them to be really sensitive. They could detect even small amounts of the different RNA transcripts produced by the minigene. This is crucial for picking up what we call leaky variants, ones that aren't completely broken, but are still significantly impaired, maybe only producing a little bit of the correct full length to transcript. Clinical interpretation needs that level of detail.
[00:06:06] Speaker A: All right, so let's get to the results. What did they find? Did these variants actually impact splicing?
[00:06:11] Speaker B: Oh, absolutely. The impact was pretty dramatic. Out of those 52 variants they tested functionally, 46 of them. That's almost 89% showed impaired splicing. And they defined impaired as at least a 10% reduction in the normal full length transcript.
[00:06:25] Speaker A: Wow, 89%. And was the impairment usually minor or.
[00:06:29] Speaker B: Often it was severe. Get this. 34 of those 46 variants caused really severe problems, leading to less than 5% of the normal full length transcript being made. I mean, functionally, those variants are essentially dead.
[00:06:41] Speaker A: Okay, clearly disrupting things. But you mentioned earlier, this genus complex. Did they see that complexity in the splicing patterns?
[00:06:48] Speaker B: Complexity is an understatement. It was more like, well, splicing chaos in some cases. The researchers ended up annotating 89 different RNA transcripts in total across all the variants they tested. 89. That number alone just highlights how difficult it is to predict these outcomes just using a computer algorithm.
[00:07:04] Speaker A: 89 transcripts. Any specific examples that really stood out?
[00:07:08] Speaker B: Definitely. They found one particular variant, C319.5GT, that just by itself produced up to 11 different RNA isoforms from the minigene. 11.
Trying to figure out the clinical meaning of potentially 11 different protein products. Well, you can see why these functional Lab tests are just essential.
[00:07:27] Speaker A: Absolutely. So what actually happened to all these different transcripts? Were they just producing junk protein?
[00:07:32] Speaker B: Well, that's the critical question. They found that 59 out of those 89 annotated transcripts introduced something called a premature termination code or a ptc. A PTC is basically an early stop signal in the genetic code.
[00:07:44] Speaker A: And the cell doesn't like those, right?
[00:07:45] Speaker B: Exactly. The cell has a quality control system called nonsense mediated decay.
If NMD spots one of these early stop codons, it usually just destroys the faulty transcript before it even gets made into protein. So finding the 59 transcripts lead to PTCs is strong evidence for pathogenicity. The gene product likely isn't being made properly or at all.
[00:08:05] Speaker A: Okay, so NMD is a major mechanism. What else did they find? Was there anything surprising about how the variants caused errors?
[00:08:11] Speaker B: Yes, something quite interesting about alternative splicing. It turns out many variants didn't actually invent completely new splicing errors. Instead, they seemed to dramatically increase the production of alternative splice forms that already exist at very low levels, even in people without the variant.
[00:08:28] Speaker A: So the mutation is kind of exploiting a pre existing weakness.
[00:08:31] Speaker B: That's a great way to put it. They identified eight such pre existing isoforms that were significantly amplified by certain mutations. It's like the gene normally has these minor alternative pathways, and the mutation just shunts everything down those faulty routes.
[00:08:45] Speaker A: Fascinating. And speaking of unexpected things, tell us about this atypical splicing site they found. The one involving the variant C6842AG.
[00:08:54] Speaker B: Ah, yes. That was a really cool piece of mechanistic discovery. This variant caused the splicing machinery to use a rare non standard TG acceptor site. And instead of the usual AG site.
[00:09:05] Speaker A: How rare are those TG sites?
[00:09:07] Speaker B: Incredibly rare. They make up only about 0.02% of all the three prime splice sites in the human genome. So finding one being actively used due to a variant is quite something. It pushed them beyond just saying splicing is broken to figuring out how it broke.
[00:09:23] Speaker A: And they pinned down the how they.
[00:09:24] Speaker B: Did, using some very precise experiments involving tiny deletions in Exon 6. They actually mapped the specific region, the C 685 to 698 interval that was necessary for the cell's machinery to even recognize and use this weird error prone TG site. Really detailed work.
[00:09:42] Speaker A: Okay, so they have all this detailed biological data. How do they translate that back into something clinically useful, like a classification?
[00:09:48] Speaker B: Right, that's the crucial next step they took. Their minigene results and plugged them directly into the standard framework used for classifying variants. The ACM JMP guidelines.
[00:09:57] Speaker A: Those are the point based guidelines everyone uses, right? Signing points for different types of evidence.
[00:10:01] Speaker B: Exactly. And strong functional evidence, like showing a variant severely disrupts splicing in. A reliable assay like this allows you to apply a specific code, PVS1, which stands for Pathogenic Variant Strong Evidence Type 1. Because this is an in vitro or observational test, they qualify it as PVS1O. Applying that code gives a lot of points towards classifying a variant as pathogenic or likely pathogenic.
[00:10:26] Speaker A: And did it work? Did they manage to reclassify many variants?
[00:10:29] Speaker B: It worked really well for a significant chunk of them. Out of the 52 variants they focused on, they successfully reclassified 32. Two ended up as pathogenic, 25 is likely pathogenic and five as likely benign. That's clear answers for almost two thirds of the variants they tested.
[00:10:44] Speaker A: That's a huge improvement over the original uncertainty. How did their classifications compare to what was already in Clinvar?
[00:10:51] Speaker B: That comparison really showed the value.
They looked at a subset of 22 variants that had conflicting or uncertain entries in Clinvar. And their new functional data allowed them to confidently reclassify eight of those. That's a 36% shift towards certainty. It was particularly effective for those tricky non canonical splice site variants, moving several VUSS into the likely pathogenic category.
[00:11:15] Speaker A: That's impressive. A real validation for using minigenes. But you said they reclassified 32 out of 52. What about the other 20? Why did nearly 40% remain as Vuss even after all this detailed functional work? Does that mean the minigenes weren't quite telling the whole story?
[00:11:30] Speaker B: Not necessarily that the minigenes were wrong, but more that the biological reality and the current classification rules are just, well, really complex. It highlights the remaining challenges.
[00:11:39] Speaker A: What kind of challenges?
[00:11:40] Speaker B: A few things. Some variants were leaky. They didn't completely abolish the normal transcript, they just reduced it maybe to 20 or 30% of the normal levels. And classifying those is tough. Is 30% function enough to prevent disease? The guidelines struggle with that ambiguity.
[00:11:55] Speaker A: Ah, okay, the gray area.
[00:11:56] Speaker B: Exactly. Plus some variants affected regions like the 5 Prime UTR, which are known to be important for gene regulation, but aren't currently well covered by the standard PVS1 criteria in the ACM JAM guidelines. So even if the Mitagene showed a clear functional effect, they couldn't always translate that into the points needed to move it out of the vus. Category based on current rules.
It shows how data generation sometimes outpaces our ability to formally interpret it.
[00:12:23] Speaker A: So, wrapping this up, what's the big picture implication here? The minogene approach seems clearly validated as a powerful tool, right?
[00:12:30] Speaker B: Absolutely. It's a fantastic method for screening potentially hundreds of these splicing variants in a relatively high throughput way.
But the study also rightly emphasizes that you still need these kinds of functional wet lab assays.
Computational predictions, even the sophisticated ones like splice AI are getting better, but they just can't fully replace seeing what actually happens in a relevant cell type.
[00:12:53] Speaker A: So prediction tools are helpful guides, but functional testing is still king for confirmation.
[00:12:58] Speaker B: Precisely. And even with great functional data, the final step for really nailing down clinical risk often still requires looking at the bigger picture, like large case control studies or family segregation data to get those precise risk estimates needed for confident clinical management.
[00:13:15] Speaker A: Okay, so the central insight here seems to be that functional assays like this minigene system are really essential tools for navigating the sort of dark matter of the genome, especially these tricky splicing mutations.
[00:13:28] Speaker B: Couldn't have said it better myself.
[00:13:29] Speaker A: Feel like the way and this deep dive certainly showed how that light successfully moved a lot of high risk sheik two variants out of that confusing clinical limbo and into categories where action can actually be taken that significantly boosts the value of genetic testing for cancer risk. Which leaves us with a final thought for you, our listener. What does this increased power of functional data mean for the future of genetic screening guidelines? If assays like these prove so effective at resolving uncertainty and guiding potentially life saving care, could we see functional evidence like that PVS 1.0 code becoming not just helpful, but maybe even a required piece of the puzzle for classifying critical variants down the line?
This episode was based on an Open Access article under the CCBY 4.0 license. You can find a direct link to the paper and the license in our episode description. If you enjoyed this, follow or subscribe in your podcast app and leave a five star rating. If you'd like to support our work, use the donation link in the description. Thanks for listening and join us next time as we explore more science base by base.
[00:14:33] Speaker B: Sam.