Show Notes
️ Episode 34: Enzyme Replacement Therapy in Homocystinuria — Safety and Efficacy of Pegtibatinase in the Phase 1/2 COMPOSE Trial
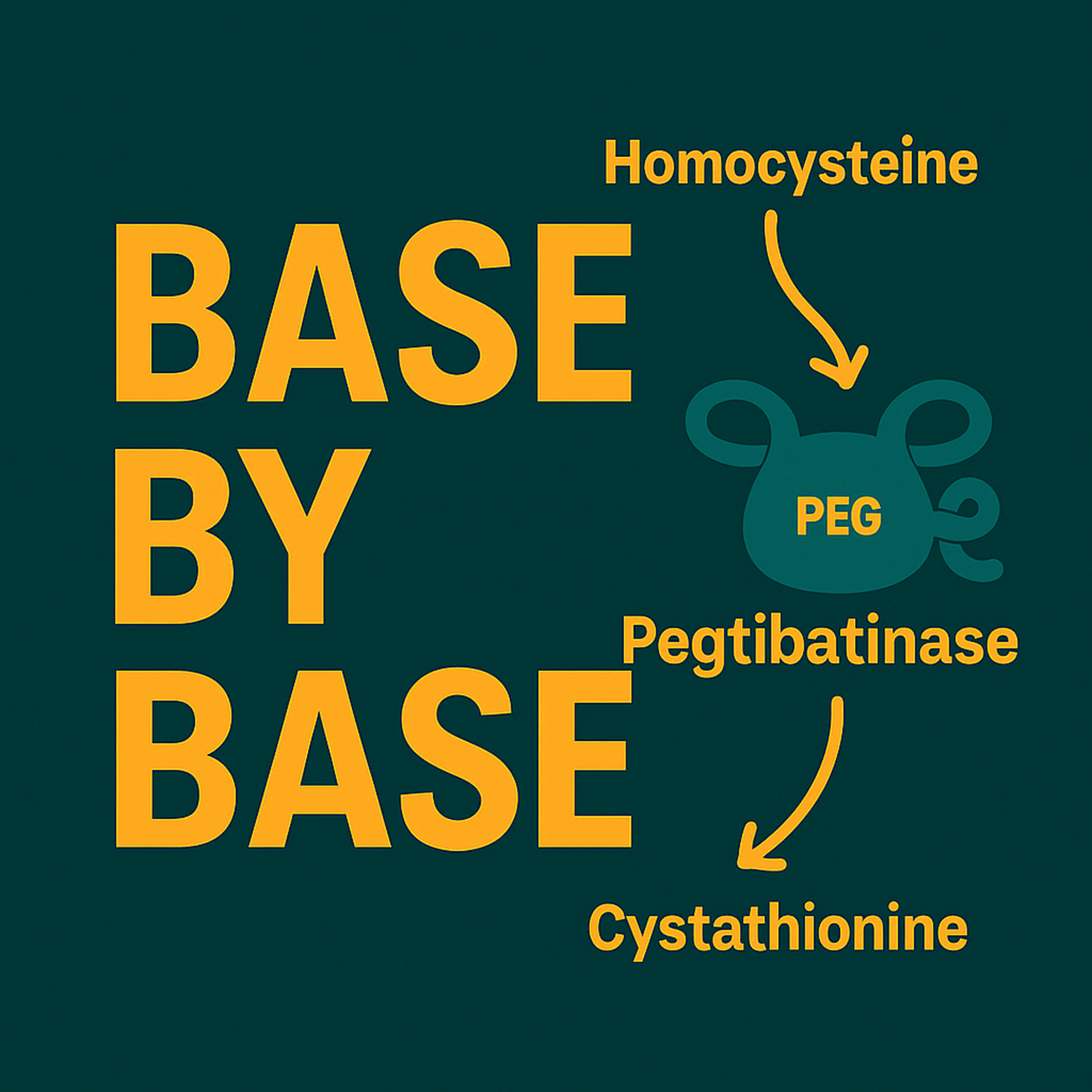
In this episode of Base by Base, we delve into early findings from Ficicioglu et al. (2025) published in Genetics in Medicine, which evaluate pegtibatinase, a PEGylated truncated human cystathionine β-synthase enzyme replacement, in adults with classical homocystinuria (HCU). The COMPOSE Phase 1/2 trial randomized individuals with elevated total plasma homocysteine (tHcy) despite standard‐of‐care treatment to receive escalating doses of subcutaneous pegtibatinase or placebo, assessing safety, immunogenicity, and metabolic outcomes .
Key highlights of the study:
The trial enrolled 24 participants aged 12–65 years across six dose cohorts, with pegtibatinase administered up to 2.5 mg/kg twice weekly and placebo controls
Pegtibatinase was generally well tolerated, with no anaphylaxis or severe immune reactions; treatment‐emergent adverse events were mostly injection‐site reactions and mild-to-moderate urticaria managed by dose interruption and premedication
Geometric mean reductions in tHcy were dose-dependent, with the highest doses (1.5 mg/kg BIW and 2.5 mg/kg BIW) achieving 57 % and 67 % relative decreases, respectively, and all patients in these cohorts maintaining tHcy below 100 μM
One participant receiving 2.5 mg/kg BIW normalized tHcy (< 15 μM) and reduced methionine (< 14 μM), enabling dietary protein liberalization without adverse events
Quantitative measurements showed restoration of methionine-cycle metabolites, including increased cysteine and cystathionine and decreased S-adenosylhomocysteine, indicating broader pathway correction
Anti-drug antibody incidence was transient and low in titer, suggesting pegtibatinase is not highly immunogenic and that antibodies did not affect pharmacokinetics
Conclusion:
The COMPOSE trial demonstrates that pegtibatinase offers a promising enzyme replacement approach for adults with classical homocystinuria, achieving substantial and sustained reductions in plasma homocysteine with an acceptable safety profile. These findings support continued investigation in larger and longer-term studies to confirm clinical benefits and explore impacts on diet and quality of life.
Reference:
Ficicioglu, C., Thomas, J. A., Ganesh, J., Kudrow, D., Lah, M., Smith, W. E., Güner, J., McDermott, S., Vaidya, S. A., Wilkening, L., & Levy, H. L. (2025). Safety and efficacy of pegtibatinase enzyme replacement therapy in adults with classical homocystinuria in the COMPOSE® phase 1/2 randomized trial. Genetics in Medicine. Advance online publication
License:
This episode summary is based on an open-access article published under the Creative Commons Attribution 4.0 International License (CC BY 4.0) – https://creativecommons.org/licenses/by/4.0/