Show Notes
️ Episode 32: Idursulfase Beta — A New Therapeutic Option for MPS II with Strong Clinical Evidence
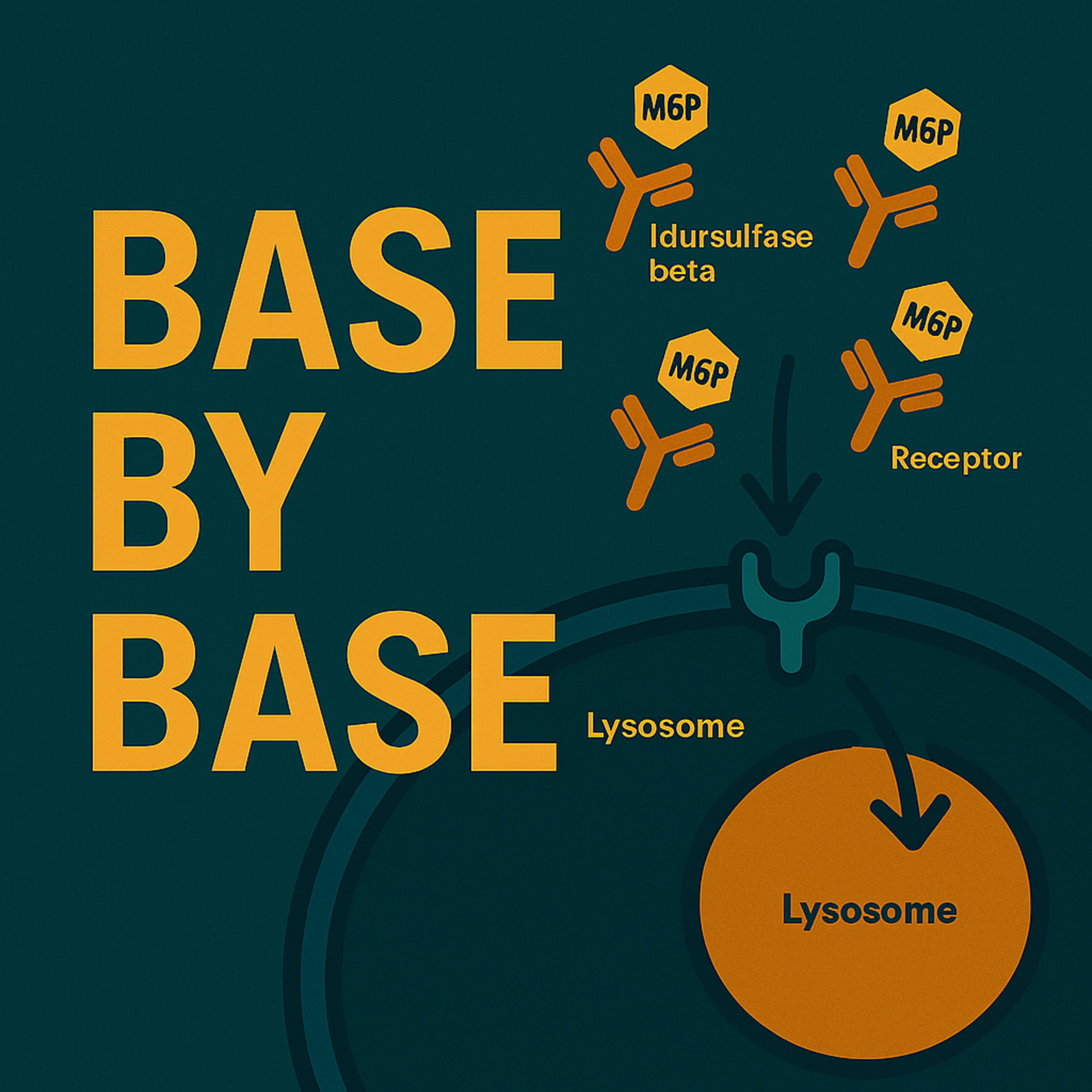
In this episode of Base por Base, we delve into a pivotal phase 3 clinical study published by Sohn et al. (2025) in Genetics in Medicine, evaluating the efficacy and safety of idursulfase beta in patients with mucopolysaccharidosis type II (MPS II), also known as Hunter syndrome. This two-part study compared idursulfase beta to a historical placebo group drawn from a prior trial with idursulfase (Elaprase®), providing compelling evidence to support its use as enzyme replacement therapy (ERT) in MPS II.
The study demonstrated that weekly intravenous treatment with idursulfase beta (0.5 mg/kg) led to significant improvements in key clinical outcomes. Participants receiving the drug showed an average increase of 62.2 meters in the six-minute walk test (6-MWT) at week 53, compared to only 7.3 meters in the historical placebo group. Additionally, urinary glycosaminoglycan (GAG) excretion dropped by more than 70%, and liver and spleen volumes were markedly reduced. These results reflect robust improvements in somatic burden and functional capacity.
The drug was well tolerated, with a favorable safety profile and lower rates of persistent neutralizing antibody formation compared to idursulfase. Notably, no serious adverse events occurred in patients with missense variants. Pharmacokinetic data confirmed stable drug exposure across the treatment period.
The use of a historical placebo group was justified by ethical concerns surrounding rare disease trials and validated by the consistent performance of idursulfase in both contemporary and historical settings. The reduction in urinary heparan and dermatan sulfate and the organ volume improvements reinforce the drug's biological efficacy.
Conclusion:
Idursulfase beta represents a safe and effective ERT alternative for MPS II, demonstrating superior clinical outcomes and reduced immunogenicity in this rigorously designed trial. These findings broaden therapeutic options for a complex and multisystemic rare disease, offering hope for better functional outcomes in affected individuals.
Reference:
Sohn, Y.B., Yang, A., Kim, M.S., et al. (2025). Efficacy and safety of idursulfase beta in the treatment of mucopolysaccharidosis II: a phase 3, two-part study compared to a historical placebo cohort. Genetics in Medicine, [online ahead of print]. https://doi.org/10.1016/j.gim.2025.101460
License:
This episode is based on an open-access article published under the Creative Commons Attribution 4.0 International (CC BY 4.0) license – https://creativecommons.org/licenses/by/4.0/